Research
Research Area: The research activities in the AKS group fall under the broad domain of inorganic and organometallic chemistry and catalysis. Our group focuses on designing new pincer and N-heterocyclic carbene ligands and their transition metal complexes. The group explores small molecule activation, C-H activation, and functionalization, aiming to develop sustainable chemical processes and renewable energy solutions.
Investigation of fundamental aspects of these activities and their mechanisms is carried out for the potential application in the development of renewable energy and sustainable chemical processes, including the following:
- CO2 capture, functionalisation, and reduction
- H2 production, storage, and transportation
- Development of Liquid Organic Hydrogen Carriers (LOHCs)
- Catalytic systems for hydrogenation/dehydrogenation reactions
- Catalytic systems for C-C, C-N, and C-O bond-forming reactions
The research integrates experimental and computational approaches, including DFT modeling, to understand reaction mechanisms and optimize catalytic performance. The key research areas can be grouped as follows:
Ligand Design and Structural Modifications: One of the most reliable ways to obtain new catalysts with novel reactivity is tuning the electronic and steric properties of the active metal centers via changes made to the ligands bound to them. Therefore, ligand design and development of new ligands and their metal complexes have become one of the most important aspects of synthetic chemistry.
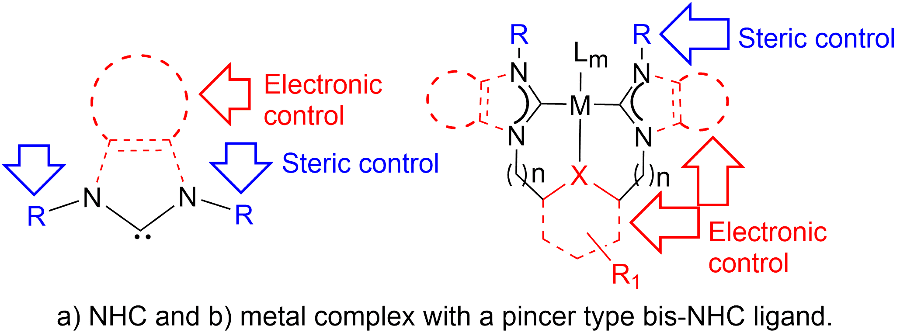
- Protic- and Classical-NHCs: Engineering phosphine-free complexes with both protic and classical NHC functionalities, analyzing the catalytic and structural implications.
- Hemilabile Ligands: Designing ligands that exhibit hemilability, enabling higher activity in catalytic cycles.
- Abnormal- vs. Normal-NHC Complexes: Exploring the reactivities of complexes containing abnormal- and normal-NHCs and investigating interconversion pathways.
Small Molecule Activation and Bond Cleavage: Understanding the cooperative small-molecule activation capabilities of Ru(II)-protic and anionic-NHC ligands, enabling selective cleavage of H-H, C-H, and other bonds.
Catalysis for Hydrogenation & Dehydrogenation: Investigating our complexes for LOHC applications towards hydrogen production, storage, and transportation.
CO₂ Capture & Functionalization: Developing catalytic systems for carbon dioxide reduction and contributing to green energy solutions.
Catalysis and Mechanistic Studies: Several of our studies explore these complexes as efficient catalysts for organic transformations, elucidating mechanisms via in situ transformations, spectroscopic characterization and DFT modeling.
