Research
Research Area: The research activities in my group fall under the broad domain of inorganic and organometallic chemistry and catalysis. My group focuses on designing new pincer and N-heterocyclic carbene ligands and their transition metal complexes capable of the following type of reactivities:
- Small molecule activation
- C-H activation and functionalisation
Investigation of fundamental aspects of these activities and their mechanisms are carried out for the potential application in the development of renewable energy and sustainable chemical processes, including the following:
- CO2 capture, functionalisation, and reduction
- H2 production, storage, and transportation
- Development of Liquid Organic Hydrogen Carriers (LOHCs)
- Catalytic systems for hydrogenation/dehydrogenation reactions
- Catalytic systems for C-C, C-N, and C-O bond-forming reactions
Ligand Design: One of the most reliable ways to obtain new catalysts with novel reactivity is tuning the electronic and steric properties of the active metal centers via changes made to the ligands bound to it. Therefore, ligand design and development of new ligands and their metal complexes have become one of the most important aspects of synthetic chemistry.
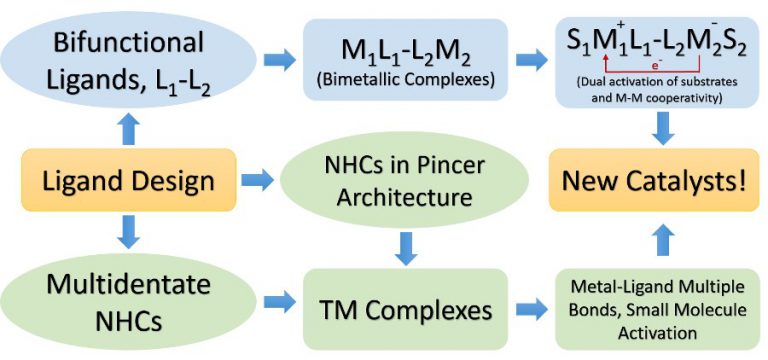
Bimetallic Systems: The idea of cooperative catalysis has inspired synthetic chemists to create artificial dual activation catalysts that use dual, cooperative activation modes, allowing for mild reaction conditions and high turnover numbers. Our group is working towards the development of a new class of bifunctional, binucleating ligands for effecting multi-electron reduction of small molecules, where the two metal centres are electronically coupled through a redox-active ligand backbone.
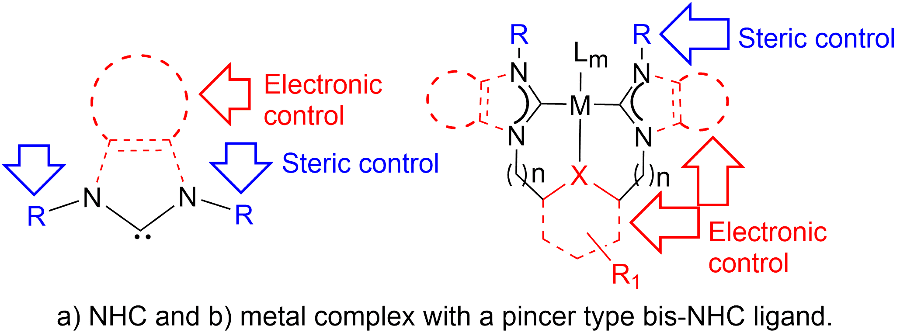
NHC & Pincer Ligands: Two ligand architectures, namely pincer ligands and tripodal chelating ligands, have been shown to provide powerful platforms for small molecule activation. Pincer ligands have been extensively investigated due to the ease by which their steric and electronic properties can be tuned. The easy tunability of NHCs facilitates systematic modifications of sterics and electronics and their integration in tripodal or pincer type ligand architectures is beneficial due to the already established robustness of such ligand systems.